Introduction
The healthcare industry's adoption of additive manufacturing (AM) marks a significant shift in medical device production. This transition from traditional manufacturing methods to AM has not only enhanced efficiency but also introduced the capability for mass personalization and the creation of complex structures in medical devices. The accompanying figure illustrates this evolution, depicting the contrast between the old and new paradigms in medical device manufacturing.
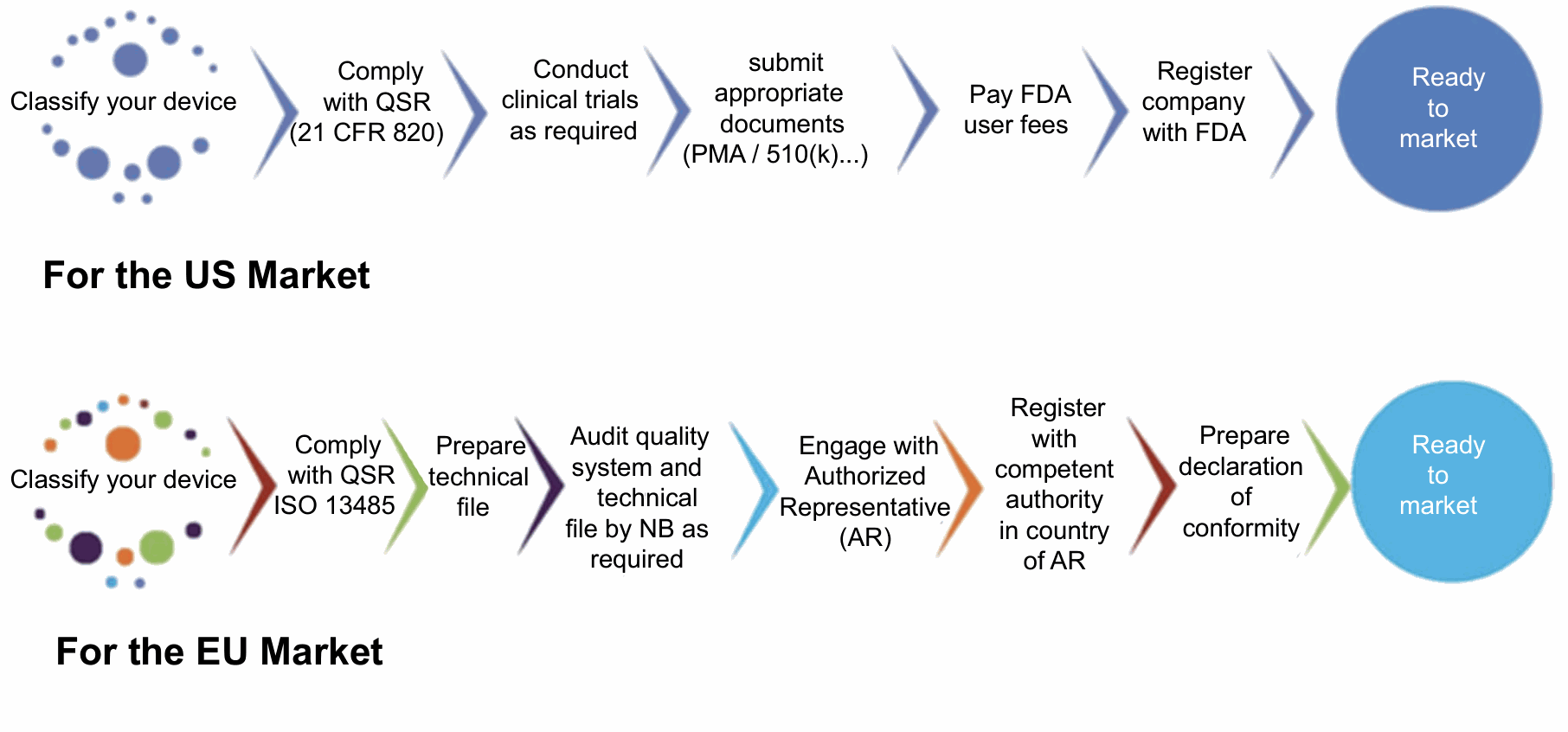
Figure: Schematic representation of steps to market medical devices in US and EU markets.
The Early Struggles
Traditional manufacturing methods, as shown in the left part of the figure, were characterized by their slow pace and high costs. These methods led to inflexible and cumbersome development cycles, often hindering innovation and responsiveness in medical device production. The figure highlights these limitations, showing the linear and restricted nature of traditional manufacturing processes.
The Rise of Additive Manufacturing
In contrast, as depicted in the right part of the figure, additive manufacturing emerged as a transformative solution. It enabled rapid prototyping, significantly reducing development time and costs. This shift allowed for greater design flexibility and faster iteration, which was particularly impactful in the production of electronic components. Technologies like the SV2 PCB printer from Botfactory played a pivotal role in this transition.
Regulatory and Standards Development
The article by Rafi et al. emphasizes that the healthcare industry's highly regulated nature necessitates a robust regulatory framework and standards for AM-produced parts. This framework ensures the safety and efficacy of these parts, which is crucial for their acceptance in healthcare. The development of such regulations and standards is an ongoing process, adapting to the evolving capabilities of AM technologies.
| Aspect | Description |
| Safety and Efficacy | AM parts must meet stringent safety and efficacy standards. |
| Regulatory Approval | Parts made by AM must be approved through a regulatory framework. |
| Standards Development | Ongoing development of standards specific to AM in healthcare. |
| Geographic Variations | Regulatory frameworks vary across different geographic domain |
Table: Regulatory Framework for Additive Manufacturing in Medical Devices
Case Study: Carilion Clinic Innovation (CCI)
Carilion Clinic Innovation (CCI) represents a pioneering force in healthcare innovation. Committed to Carilion’s mission of enhancing community health, CCI harnesses the expertise of doctors, nurses, and clinical professionals to transform ideas into tangible solutions. This interdisciplinary team of engineers, human factors specialists, and medical experts, collaborates to identify system requirements and brainstorm solutions. Central to their innovation process is the Makerspace, which is equipped with 3D printers, a laser cutter, and notably, the Botfactory PCB printer. The SV2 PCB printer from Botfactory has been instrumental in elevating CCI's productivity. It enables the team to swiftly create and test electronic components and circuitry, significantly accelerating the design process. The printer's capability to fabricate circuits within hours allows for rapid design improvements, a feat unattainable with traditional methods.
The image on the right: Carilion Clinic Innovation Engineer Connor Hale preparing the SV2 for his research activities.

Further enhancing their development process, Carilion’s Center for Simulation, Research and Patient Safety (Sim Center) offers realistic mockups of ORs, ICUs, and other hospital settings. These high-fidelity environments are crucial for prototype testing, providing immediate and relevant feedback from clinicians who will use the devices. This approach streamlines product development and ensures that the end products closely align with the actual needs of patients and clinicians. The result is a range of innovative medical devices that contribute to better health outcomes and cost efficiencies, demonstrating the transformative impact of additive manufacturing in the medical field.
Conclusion
The transformative journey of medical hardware, propelled by the advent of additive manufacturing, marks a significant milestone in healthcare technology. This evolution from traditional, cumbersome manufacturing methods to the agile, efficient capabilities of additive manufacturing has revolutionized the way medical devices are conceived, designed, and produced. The case of Carilion Clinic Innovation underscores the practical applications of these advancements, demonstrating how rapid prototyping and testing, enabled by technologies like the SV2 PCB printer from Botfactory, can lead to innovative medical solutions. These developments not only enhance the quality and functionality of medical devices but also contribute to improved patient outcomes and cost efficiencies. As the medical industry continues to embrace and integrate these technological advancements, the future of medical device development is poised to be more dynamic, patient-centric, and responsive to the evolving needs of healthcare.
References
- Regulatory and standards development in medical additive manufacturing. Journal of Materials Research and Technology.
- Fully-Printed Piezoelectric Devices for Flexible Electronics Applications.” Advanced Materials Technologies.
- Flexible Electronics and Devices as Human–Machine Interfaces for Medical Robotics.” Journal of Materials Research.
